The Oncogenic Helicase ALC1 Regulates PARP Inhibitor Potency by Trapping PARP2 at DNA Breaks
2020-10-29
Charlotte Blessing, Imke Karlijn Mandemaker, Claudia Gonzalez-Leal, Julia Preisser, Adrian Schomburg and Andreas Gerhard Ladurner
Molecular Cell, 2020, 80, 1–14
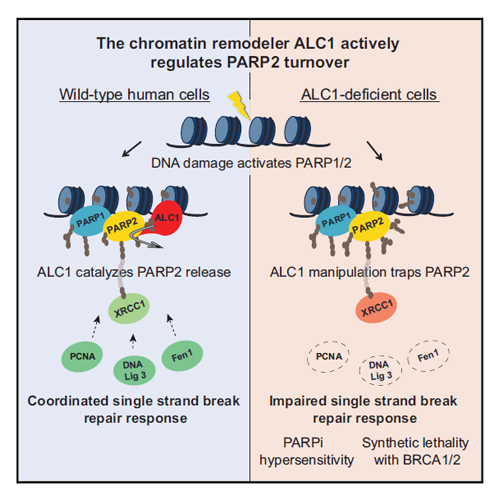
The anti-tumor potency of poly(ADP-ribose) polymerase (PARP) inhibitors (PARPis) has been linked to trapping of PARP1 on damaged chromatin. However, little is known about their impact on PARP2, an isoform with overlapping functions at DNA lesions. Whether the release of PARP1/2 from DNA lesions is actively catalyzed by molecular machines is also not known. We found that PARPis robustly trap PARP2 and that the helicase ALC1 (CHD1L) is strictly required for PARP2 release. Catalytic inactivation of ALC1 quantitatively traps PARP2 but not PARP1. ALC1 manipulation impacts the response to single-strand DNA breaks through PARP2 trapping, potentiates PARPi-induced cancer cell killing, and mediates synthetic lethality upon BRCA deficiency. The chromatin remodeler ALC1 actively drives PARP2 turnover from DNA lesions, and PARP2 contributes to the cellular responses of PARPi. This suggests that disrupting the ATP-fueled remodeling forces of ALC1 might enable therapies that selectively target the DNA repair functions of PARPs in cancer.