Aza-SAHA Derivatives Are Selective Histone Deacetylase 10 Chemical Probes That Inhibit Polyamine Deacetylation and Phenocopy HDAC10 Knockout
2022-10-06
Raphael R. Steimbach, Corey J. Herbst-Gervasoni, Severin Lechner, Tracy Murray Stewart, Glynis Klinke, Johannes Ridinger, Magalie N. E. Géraldy, Gergely Tihanyi, Jackson R. Foley, Ulrike Uhrig, Bernhard Kuster, Gernot Poschet, Robert A. Casero, Jr., Guillaume Médard, Ina Oehme, David W. Christianson, Nikolas Gunkel, and Aubry K. Miller
J. Am. Chem. Soc. 2022, 144, 41, 18861–18875, 2022
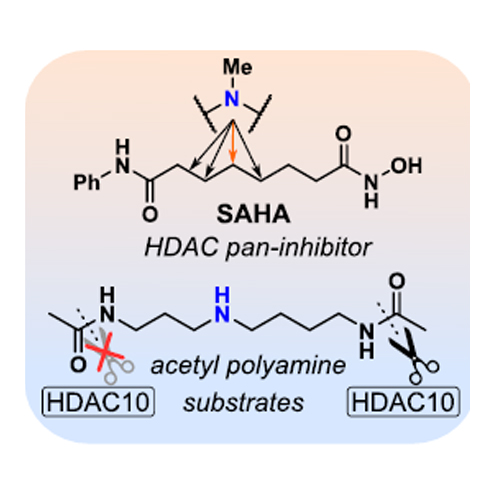
We report the first well-characterized selective chemical probe for histone deacetylase 10 (HDAC10) with unprecedented selectivity over other HDAC isozymes. HDAC10 deacetylates polyamines and has a distinct substrate specificity, making it unique among the 11 zinc-dependent HDAC hydrolases. Taking inspiration from HDAC10 polyamine substrates, we systematically inserted an amino group (“aza-scan”) into the hexyl linker moiety of the approved drug Vorinostat (SAHA). This one-atom replacement (C→N) transformed SAHA from an unselective pan-HDAC inhibitor into a specific HDAC10 inhibitor. Optimization of the aza-SAHA structure yielded the HDAC10 chemical probe DKFZ-748, with potency and selectivity demonstrated by cellular and biochemical target engagement, as well as thermal shift assays. Cocrystal structures of our aza-SAHA derivatives with HDAC10 provide a structural rationale for potency, and chemoproteomic profiling confirmed exquisite cellular HDAC10-selectivity of DKFZ-748 across the target landscape of HDAC drugs. Treatment of cells with DKFZ-748, followed by quantification of selected polyamines, validated for the first time the suspected cellular function of HDAC10 as a polyamine deacetylase. Finally, in a polyamine-limiting in vitro tumor model, DKFZ-748 showed dose-dependent growth inhibition of HeLa cells. We expect DKFZ-748 and related probes to enable further studies on the enigmatic biology of HDAC10 and acetylated polyamines in both physiological and pathological settings.