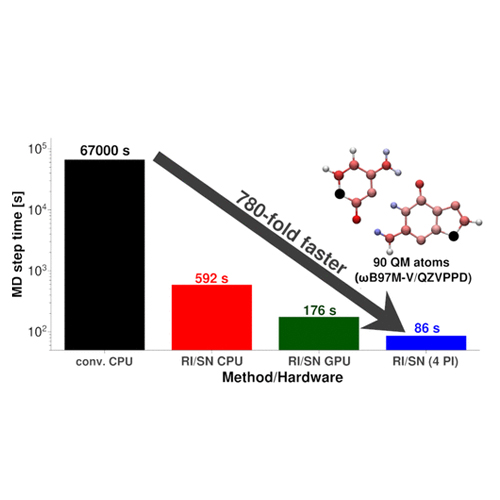
How to obtain reaction free energies from free-energy profiles
2022-03-15
Johannes C. B. Dietschreit, Dennis J. Diestler, Christian Ochsenfeld
J. Chem. Phys. 156, 114105, 2022
For chemical reactions that occur via the rearrangement of atoms from a configuration about one minimum (reactant, R) of the potential energy surface (PES) to a configuration about another minimum (product, P), an exact relation between the Helmholtz reaction free energy (ΔFRP) and the free-energy profile (FEP) can be derived. Since the FEP assumes a form similar to that of the PES along the minimum energy path between R and P, there is an unfortunate tendency to regard the FEP as the “free-energy” analog of the minimum energy path and consequently to equate ΔFRP to the difference between the values of the FEP at the minima corresponding to R and P. Analytic treatments of one- and two-dimensional models are presented that show how this mistaken idea leads to errors. In effect, treating the FEP by analogy with the minimum energy path neglects the role of entropy. The FEP is a function of a collective variable (CV), which must be chosen to describe the course of the rearrangement consistently with the exact relation between ΔFRP and the FEP. For large systems of common interest, the PES is often so complex that a straightforward way of choosing a CV is lacking. Consequently, one is forced to make an educated guess. A criterion for judging the quality of the guess is proposed and applied to a two-dimensional model.