Cu-Catalyzed Azide−Alkyne−Thiol Reaction Forms Ubiquitous Background in Chemical Proteomic Studies
2024-01-12
Andreas Wiest and Pavel Kielkowski
J. Am. Chem. Soc, 2024
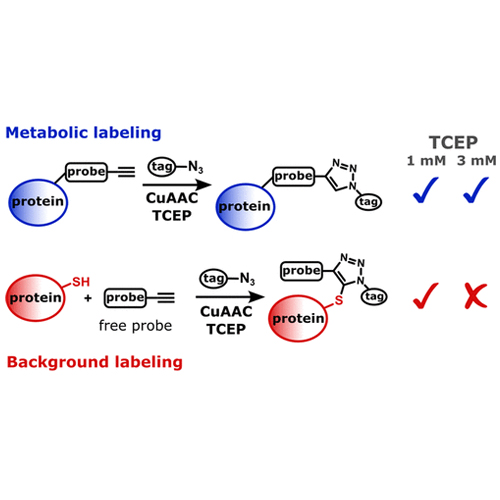
We report here a Cu-catalyzed azide–alkyne–thiol reaction forming thiotriazoles as the major byproduct under widely used bio-orthogonal protein labeling “click” conditions. The development of Cu(I)-catalyzed azide–alkyne cycloaddition (CuAAC) had a tremendous impact on many biological discoveries. However, the considered chemoselectivity of CuAAC is hampered by the high reactivity of cysteine free thiols, yielding thiotriazole protein conjugates. The reaction byproducts generate false-positive protein hits in functional proteomic studies. The reported detail investigation of conjugates between chemical probes containing terminal alkynes, azide tags, and cell lysates reveals the formation of thiotriazoles, which can be readily detected by in-gel fluorescence scanning or after peptide and protein enrichment by mass spectrometry-based proteomics. In protein level identification and quantification experiments, the produced fluorescent bands or enriched proteins may not result from the important enzymatically driven reaction and can be falsely assigned as hits. This study provides a complete list of the most common background proteins. The knowledge of this previously overlooked reactivity now leads to the introduction of modified CuAAC conditions, which avoids the undesired product formation, diminishes the background, and hence improves the signal-to-noise ratio.