A Specialized Polythioamide-Binding Protein Confers Antibiotic Self-Resistance in Anaerobic Bacteria
2022-07-19
Finn Gude, Evelyn M. Molloy, Therese Horch, Maria Dell, Kyle L. Dunbar, Jana Krabbe, Michael Groll and Christian Hertweck
Angew. Chem. Int. Ed. 2022, e202206168
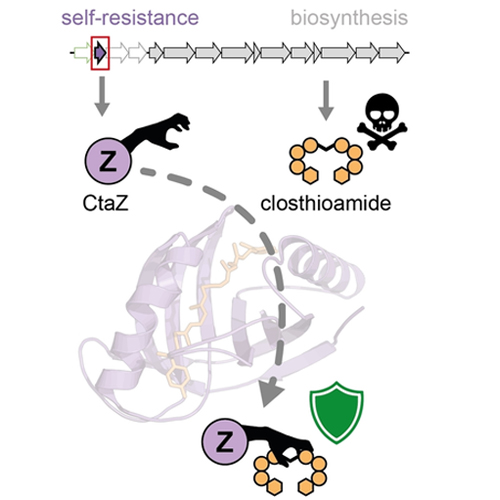
Understanding antibiotic resistance mechanisms is central to the development of anti-infective therapies and genomics-based drug discovery. Yet, many knowledge gaps remain regarding the resistance strategies employed against novel types of antibiotics from less-explored producers such as anaerobic bacteria, among them the Clostridia. Through the use of genome editing and functional assays, we found that CtaZ confers self-resistance against the copper chelator and gyrase inhibitor closthioamide (CTA) in Ruminiclostridium cellulolyticum. Bioinformatics, biochemical analyses, and X-ray crystallography revealed CtaZ as a founding member of a new group of GyrI-like proteins. CtaZ is unique in binding a polythioamide scaffold in a ligand-optimized hydrophobic pocket, thereby confining CTA. By genome mining using CtaZ as a handle, we discovered previously overlooked homologs encoded by diverse members of the phylum Firmicutes, including many pathogens. In addition to characterizing both a new role for a GyrI-like domain in self-resistance and unprecedented thioamide binding, this work aids in uncovering related drug-resistance mechanisms.