Synthesis and Incorporation of k2U into RNA
2020-02-18
Milda Nainyte, Thomas Carell
Helv. Chim. Acta, 2020, 103 (3) e2000016
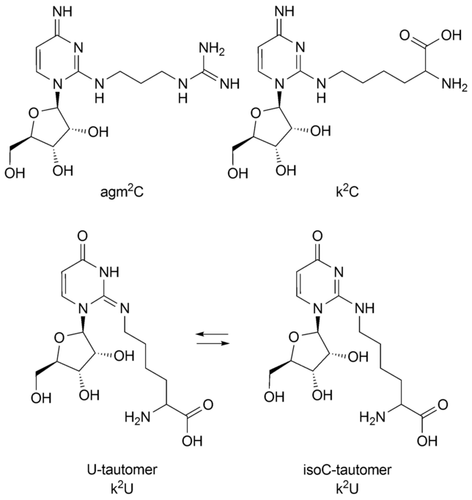
Lysidine (k2C) is one of the most modified pyrimidine RNA bases. It is a cytidine nucleoside, in which the 2‐oxo functionality of the heterocycle is replaced by the ϵ‐amino group of the amino acid lysine. As such, lysidine is an amino acid‐containing RNA nucleoside that combines directly genotype (C‐base) with phenotype (lysine amino acid). This makes the compound particularly important in the context of theories about the origin of life and here especially for theories that target the origin of translation. Here, we report the total synthesis of the U‐derivative of lysidine (k2U), which should have the same base pairing characteristics as k2C if it exists in the isoC‐like tautomeric form. To investigate this question, we developed a phosphoramidite building block for k2U, which allows its incorporation into RNA strands. Within RNA, k2U can base pair with the counter base U and isoG, confirming that k2U prefers an isoC‐like tautomeric structure that is also known to dominate for k2C. The successful synthesis of a k2U phosphoramidite and its use for RNA synthesis now paves the way for the preparation of a k2C phosphoramidite and RNA strands containing k2C.