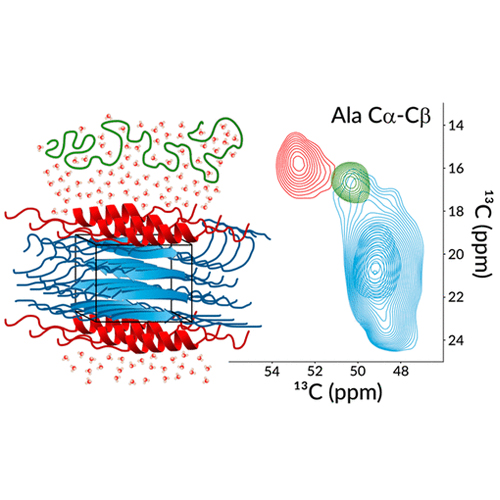
Unveiling the Dynamic Self-Assembly of a Recombinant Dragline-Silk-Mimicking Protein
2024-02-11
Dongqing Wu, Anamaria Koscic, Sonja Schneider, Romeo C. A. Dubini, Diana C. Rodriguez Camargo, Sabine Schneider, and Petra Rovó
Biomacromolecules, 2024
Despite the considerable interest in the recombinant production of synthetic spider silk fibers that possess mechanical properties similar to those of native spider silks, such as the costeffectiveness, tunability, and scalability realization, is still lacking. To address this long-standing challenge, we have constructed an artificial spider silk gene using Golden Gate assembly for the recombinant bacterial production of dragline-mimicking silk, incorporating all the essential components: the N-terminal domain, a 33-residue-long majorampullate-spidroin-inspired segment repeated 16 times, and the Cterminal domain (N16C). This designed silk-like protein was successfully expressed in Escherichia coli, purified, and cast into films from formic acid. We produced uniformly 13C−15N-labeled N16C films and employed solid-state magic-angle spinning nuclear magnetic resonance (NMR) for characterization. Thus, we could demonstrate that our bioengineered silk-like protein self-assembles into a film where, when hydrated, the solvent-exposed layer of the rigid, β-nanocrystalline polyalanine core undergoes a transition to an α-helical structure, gaining mobility to the extent that it fully dissolves in water and transforms into a highly dynamic random coil. This hydration-induced behavior induces chain dynamics in the glycine-rich amorphous soft segments on the microsecond time scale, contributing to the elasticity of the solid material. Our findings not only reveal the presence of structurally and dynamically distinct segments within the film’s superstructure but also highlight the complexity of the self-organization responsible for the exceptional mechanical properties observed in proteins that mimic dragline silk. Despite the considerable interest in the recombinant production of synthetic spider silk fibers that possess mechanical properties similar to those of native spider silks, such as the costeffectiveness, tunability, and scalability realization, is still lacking. To address this long-standing challenge, we have constructed an artificial spider silk gene using Golden Gate assembly for the recombinant bacterial production of dragline-mimicking silk, incorporating all the essential components: the N-terminal domain, a 33-residue-long majorampullate-spidroin-inspired segment repeated 16 times, and the Cterminal domain (N16C). This designed silk-like protein was successfully expressed in Escherichia coli, purified, and cast into films from formic acid. We produced uniformly 13C−15N-labeled N16C films and employed solid-state magic-angle spinning nuclear magnetic resonance (NMR) for characterization. Thus, we could demonstrate that our bioengineered silk-like protein self-assembles into a film where, when hydrated, the solvent-exposed layer of the rigid, β-nanocrystalline polyalanine core undergoes a transition to an α-helical structure, gaining mobility to the extent that it fully dissolves in water and transforms into a highly dynamic random coil. This hydration-induced behavior induces chain dynamics in the glycine-rich amorphous soft segments on the microsecond time scale, contributing to the elasticity of the solid material. Our findings not only reveal the presence of structurally and dynamically distinct segments within the film’s superstructure but also highlight the complexity of the self-organization responsible for the exceptional mechanical properties observed in proteins that mimic dragline silk.